Encapsulate Approach
The success rate of oncology drug development is notoriously low, with a majority of drug candidates failing to make it to market. According to a study published in the journal Nature Biotechnology in 2020, the overall success rate for oncology drug development is approximately 3.4%. The study also found that the success rate varies depending on the phase of clinical trials, with Phase 3 trials having the highest success rate at 32.2%, followed by Phase 2 trials at 13.2%, and Phase 1 trials having the lowest success rate at 8.4%. The high failure rate of oncology drug development is due to a number of factors, including the complex and heterogeneous nature of cancer, the difficulty in identifying and validating new drug targets, and the challenges in designing effective therapies that can selectively target cancer cells while sparing normal cells. Additionally, the high variability in patient response to treatment further adds to the difficulty in developing successful oncology drugs. Encapsulate products and services tackle all of these challenges.
According to a study conducted by the Tufts Center for the Study of Drug Development and published in the Journal of Health Economics in 2016, the estimated average cost of developing a new cancer drug from discovery to approval is approximately $2.6 billion. This figure accounts for various expenses, including the cost of capital for investors, the opportunity cost of research and development activities, and the cost of failed drug candidates. Another study published by the Institute for Clinical and Economic Review (ICER) in the Journal of Managed Care & Specialty Pharmacy in 2020 estimated the cost of cancer drug development to be around $3.2 billion.

Strategies that can help reduce the costs of oncology drug development:
Increased efficiency in drug discovery
By using computational methods, such as machine learning and artificial intelligence, drug discovery can be accelerated, and drug candidates can be identified more efficiently.
Improved preclinical testing
Preclinical testing, which includes in vitro and animal studies, can be improved by using more predictive models and biomarkers that can better predict clinical outcomes.
Adaptive trial designs
Adaptive trial designs allow for modifications to the trial design and patient population during the course of the trial, which can help reduce the number of patients needed and the duration of the trial.
Increased use of real-world evidence
Real-world evidence, such as data from electronic health records and patient registries, can be used to supplement clinical trial data and provide additional insights into drug safety and effectiveness.
Increased collaboration and data sharing
Collaboration between industry, academia, and regulatory agencies can help reduce duplication of effort and facilitate sharing of data, resources, and expertise.
Using precise pre-clinical models
With Encapsulate devices, comprehensive biobank, and custom-made ex vivo studies, industrial partners can receive a miniature model of the actual clinical trials.
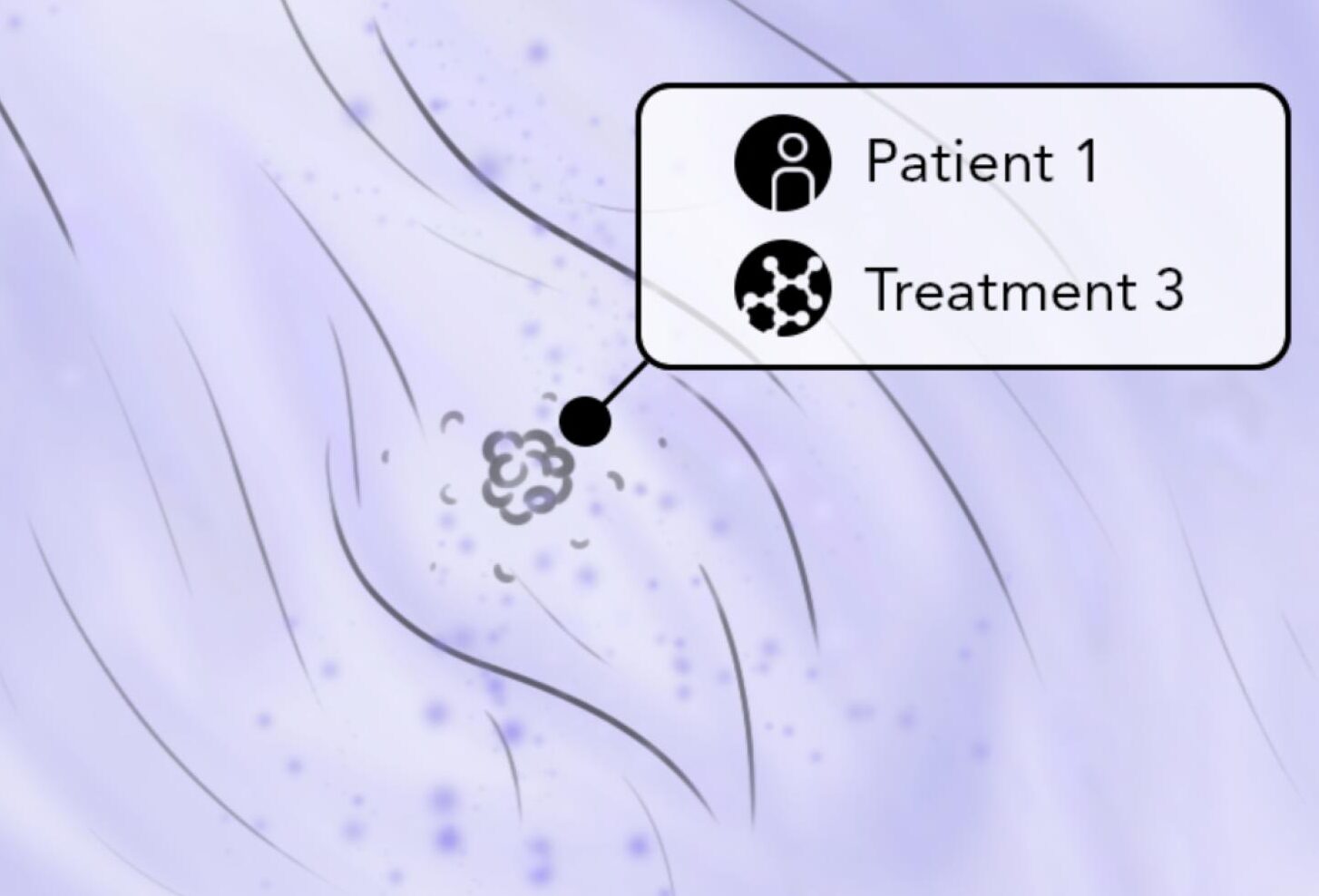
Patient stratification in a clinical trial involves grouping patients based on certain predetermined criteria, such as disease subtype, biomarker profile, or other clinical characteristics. This process is designed to identify specific subgroups of patients who may be more likely to respond to the treatment being tested in the clinical trial.
Patient stratification can be performed using a variety of methods, including genetic testing, imaging, or clinical assessment. For example, in an oncology clinical trial, patients may be stratified based on their tumor histology, mutation status, or biomarker expression levels.
By stratifying patients in this manner, the clinical trial can potentially identify patient subgroups who are more likely to benefit from the treatment being tested, and improve the statistical power of the trial by reducing the heterogeneity of the patient population. This can lead to the identification of more effective treatments in a shorter time frame, potentially improving patient outcomes and reducing the overall cost of drug development.
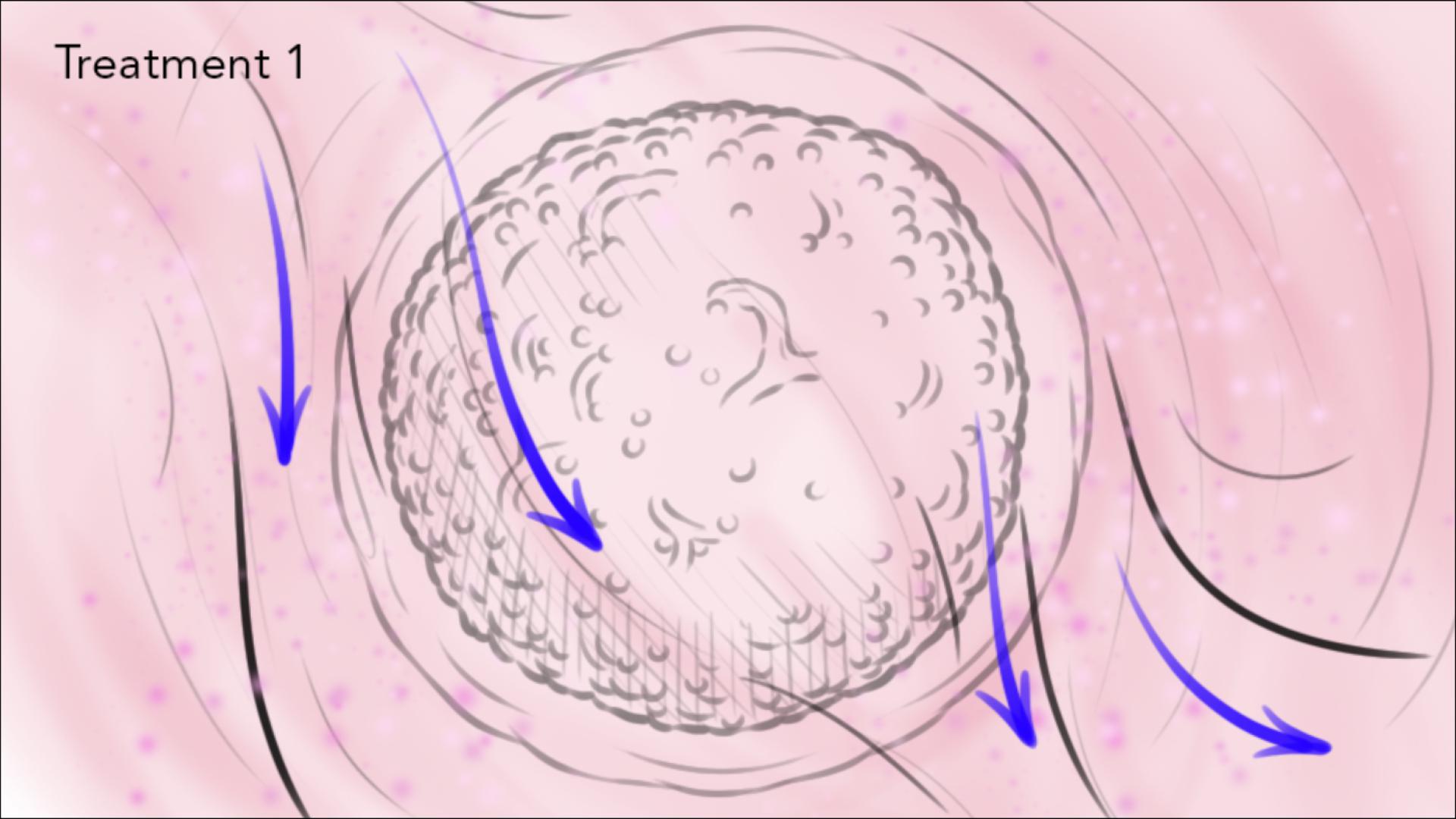
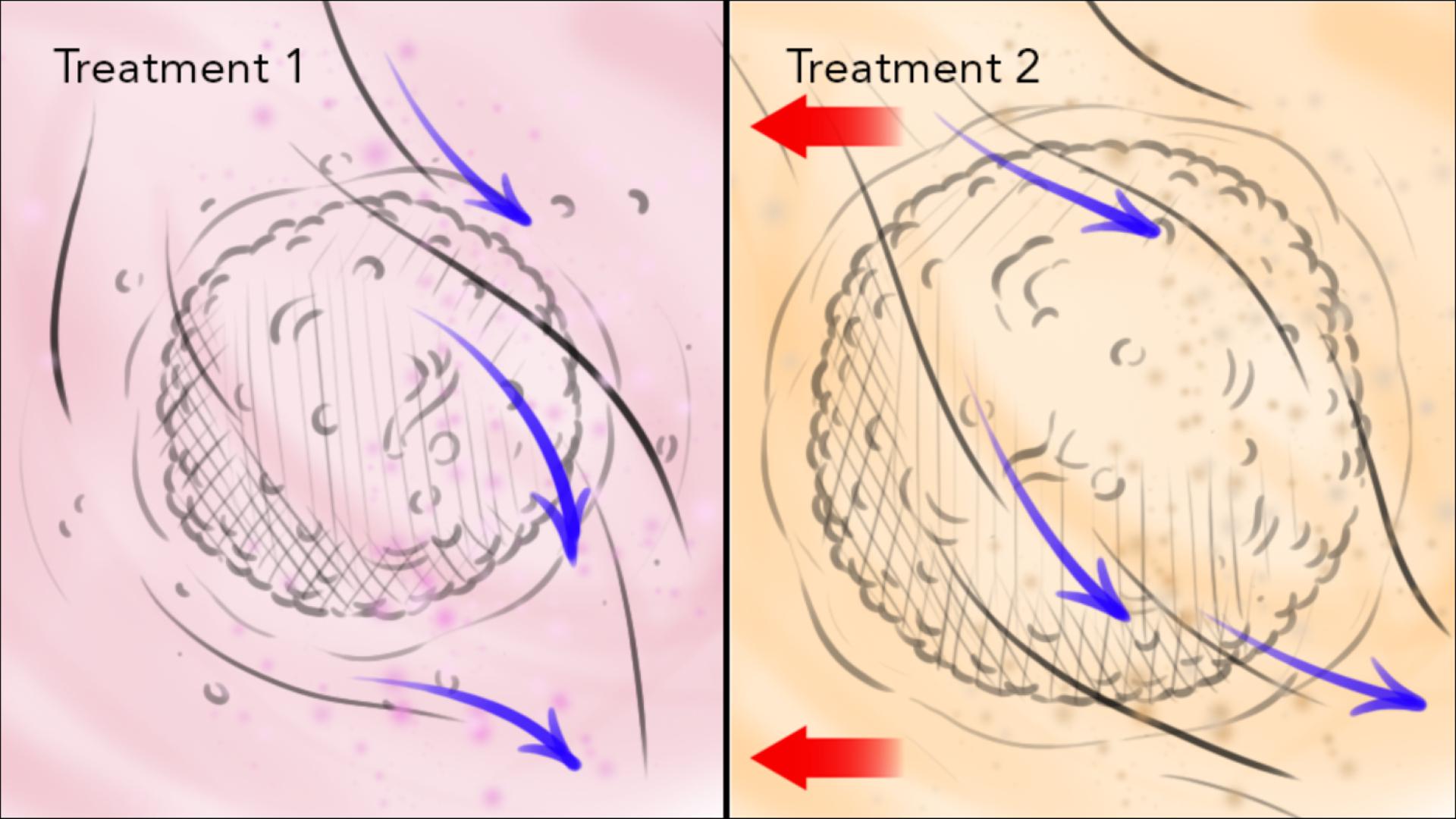
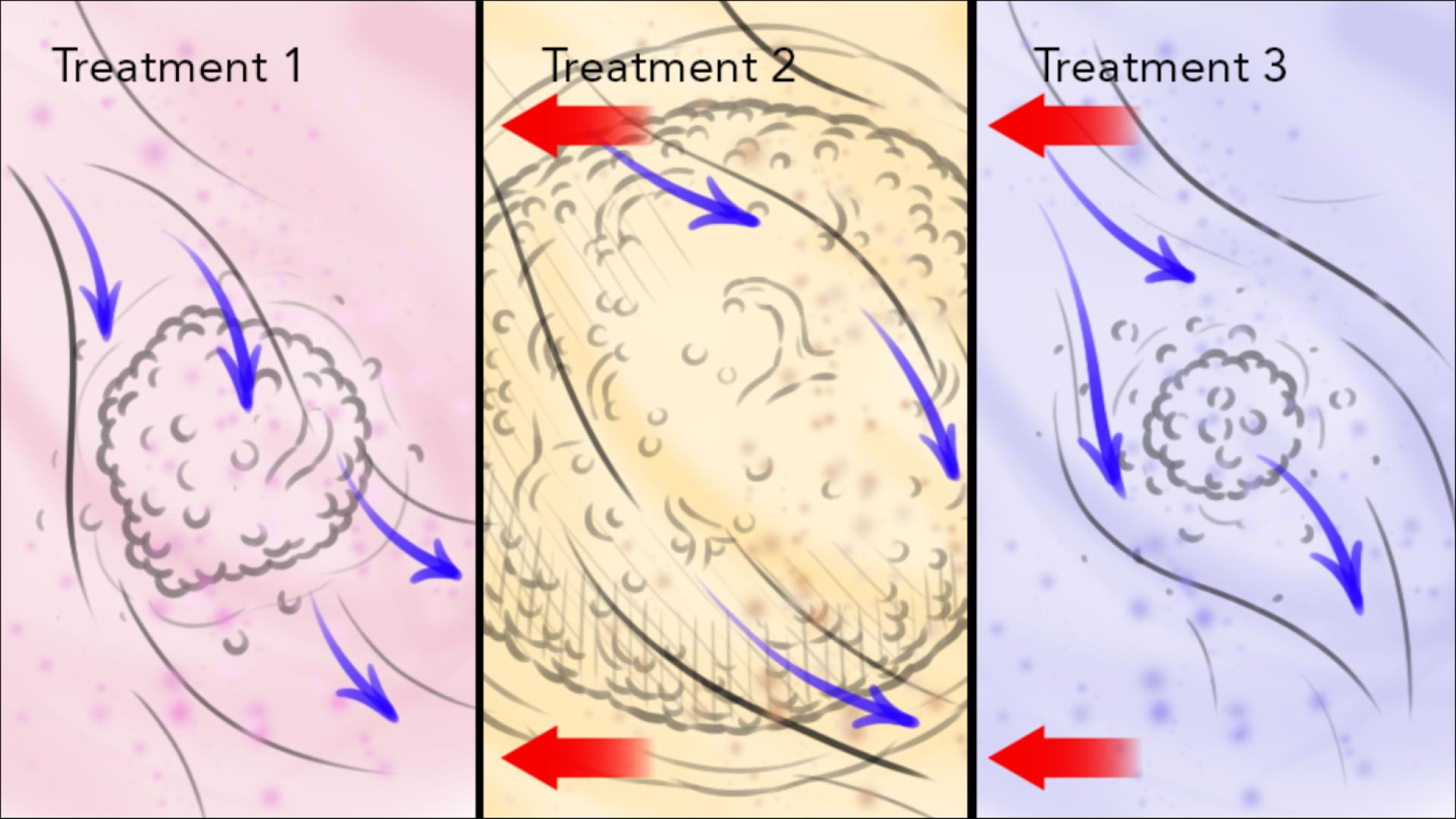
A pre-clinical trial ex vivo study is a laboratory-based investigation that is conducted on biological specimens or tissues outside of the living organism. These studies are conducted to evaluate the safety and efficacy of a drug or therapy, and to provide initial evidence of its potential effects in vivo. Ex vivo studies can be performed using various biological materials, including cells, tissues, or organs, and involve treating the material with a drug or therapy and observing its effects on cellular and molecular processes.
Pre-clinical ex vivo studies can provide important information about a drug’s pharmacokinetics, pharmacodynamics, and potential toxic effects. These studies can also help identify optimal dosing regimens and treatment protocols for subsequent in vivo studies. Overall, pre-clinical ex vivo studies are essential in drug development, providing critical insights into the safety and efficacy of potential therapies before they are tested in living organisms in clinical trials.
Patient stratification can be performed using a variety of methods, including genetic testing, imaging, or clinical assessment. For example, in an oncology clinical trial, patients may be stratified based on their tumor histology, mutation status, or biomarker expression levels. By stratifying patients in this manner, the clinical trial can potentially identify patient subgroups who are more likely to benefit from the treatment being tested, and improve the statistical power of the trial by reducing the heterogeneity of the patient population. This can lead to the identification of more effective treatments in a shorter time frame, potentially improving patient outcomes and reducing the overall cost of drug development.
nCapsule 3D system
Three-dimensional (3D) cell culture systems have been shown to provide several advantages over traditional two-dimensional (2D) cell culture systems. Firstly, they better mimic the in vivo microenvironment of tissues, which is complex and three-dimensional. This allows researchers to study cellular behavior, disease progression, and drug responses in a more physiologically relevant model. Secondly, 3D cell culture systems facilitate improved cell-cell and cell-matrix interactions, which can lead to improved cellular differentiation, cell signaling, and gene expression. Thirdly, 3D cell culture systems provide more accurate predictions of drug efficacy and toxicity compared to 2D systems, as they better represent the in vivo tumor microenvironment and its response to drugs. Additionally, some cells have a limited lifespan in 2D cultures, but can be maintained for longer periods of time in 3D cultures, allowing for more long-term experiments and assays. Lastly, some cells require a 3D microenvironment to differentiate and function properly, and cannot be accurately modeled in 2D cultures. Overall, 3D cell culture systems provide a more biologically relevant platform for studying cellular behavior, disease progression, and drug responses, and are increasingly being adopted by researchers in a variety of fields.
Advantages of nCapsule hybrid technology
More physiological relevance: nCapsule systems can better mimic the complex in vivo tumor microenvironment, including the presence of blood vessels, immune cells, and other components, which can affect tumor growth, invasion, and response to therapy.
Ability to model drug delivery and response: nCapsule systems can be designed to include microfluidic channels that allow researchers to study how drugs diffuse and interact with tumor cells in a more realistic manner.
Better control over experimental conditions: nCapsule systems allow for precise control over the microenvironment, including temperature, oxygen levels, and fluid flow, which can be important factors affecting tumor growth and response to therapy.
Higher throughput and scalability: nCapsule systems can be designed to include multiple channels or compartments, allowing for higher throughput and scalability compared to spheroid systems.
High-throughput data screening
Encapsulate devices can investigate up to 120 tumoroids in parallel.
Patient-mimicked structure
Our multi-level complex tissue model resembles the innate tumor structure and represents the natural tumor complexity & resistance.
Variety of tests
We can test any chemotherapy, radiotherapy, hormonal therapy, and targeted therapy agent.
Patient stratification
With our comprehensive patient tumor biobank, we can help you to pick the most suitable patient pool for your newly developed cancer drug.
Free consultation on the services available
Contact us
News & Updates
Leila Daneshmandi, Co-Founder and COO of Encapsulate Wins 2023 TiE Boston Women Pitch Competition
BOSTON—TiE Boston announced the winners of their highly-anticipated, annual TiE Boston Women’s Pitch Competition, which was held at PTC Headquarters in Boston’s Seaport. Leila Daneshmandi, Co-Founder and COO of Encapsulate won the first prize and will go on to...
NSF awarded Encapsulate its SBIR Phase I
News & Events Live Events Subscribe For the Latest news & Updates
Pilot Clinical Studies Completed
News & Events Live Events Subscribe For the Latest news & Updates

Get in Touch. Get Involved.
Vivamus suscipit tortor eget felis porttitor volutpat. Nulla quis lorem ut libero malesuada feugiat. Vivamus suscipit tortor eget felis porttitor volutpat. Proin eget tortor risus.