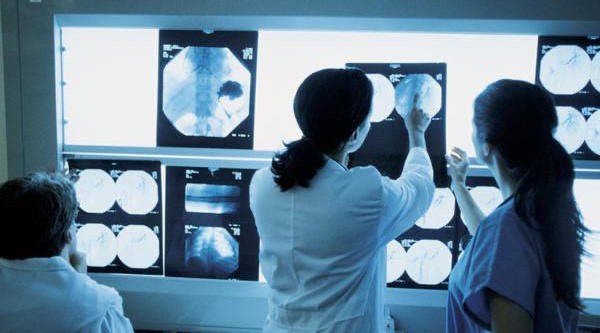
Breast cancer in perspective
Brief overview on
Understanding Triple Negative Breast Cancer: A Comprehensive Guide for Patients
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that is more aggressive and less responsive to standard treatments compared to other types of breast cancer. TNBC accounts for 10-20% of all breast cancer cases and is more common in women under the age of 50 and in African American women. The 5-year survival rate for TNBC is lower compared to other subtypes of breast cancer, at around 77% for stage I and II, and around 43% for stage III and IV. TNBC is more likely to recur within the first few years after treatment compared to other subtypes of breast cancer.
Be aware
Symptoms of breast cancer
- A lump or mass in the breast or underarm
- Swelling, redness, or warmth in the breast
- Nipple changes, such as inversion, discharge, or scaling
- Pain in the breast or nipple area
- Changes in breast size or shape
Diagnosis process
Detection of breast cancer
TNBC is diagnosed through a combination of physical exams, imaging tests, and biopsies. Imaging tests may include mammograms, ultrasounds, and MRIs. A biopsy, which involves removing a small sample of breast tissue for examination, is necessary to determine the subtype of breast cancer.
TNBC is an aggressive subtype of breast cancer that lacks estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. TNBC tumors are genetically heterogeneous, and therefore, treatment options are limited.
To develop better treatment options for TNBC, cancer researchers can leverage the common genetic mutations present in TNBC. One such mutation is in the tumor suppressor gene, TP53. Mutations in TP53 are present in up to 80% of TNBC cases, making it an attractive target for new therapies. Researchers can explore the use of small molecules and gene therapies to target TP53 and restore its tumor-suppressive function.
Another potential target for TNBC is the protein PARP1, which is involved in DNA repair. Mutations in genes involved in DNA repair, such as BRCA1 and BRCA2, can lead to the development of breast cancer. PARP inhibitors, which are already approved for the treatment of BRCA-mutated ovarian and breast cancers, are being investigated as a treatment option for TNBC patients with defects in DNA repair.
Furthermore, researchers can explore the use of immunotherapies for TNBC. Several clinical trials are currently investigating the efficacy of immune checkpoint inhibitors, such as pembrolizumab and atezolizumab, for the treatment of TNBC. These drugs work by blocking the interaction between cancer cells and immune cells, thereby enabling the immune system to recognize and attack cancer cells.
TNBC is an aggressive subtype of breast cancer that lacks estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. TNBC tumors are genetically heterogeneous, and therefore, treatment options are limited.
To develop better treatment options for TNBC, cancer researchers can leverage the common genetic mutations present in TNBC. One such mutation is in the tumor suppressor gene, TP53. Mutations in TP53 are present in up to 80% of TNBC cases, making it an attractive target for new therapies. Researchers can explore the use of small molecules and gene therapies to target TP53 and restore its tumor-suppressive function.
Another potential target for TNBC is the protein PARP1, which is involved in DNA repair. Mutations in genes involved in DNA repair, such as BRCA1 and BRCA2, can lead to the development of breast cancer. PARP inhibitors, which are already approved for the treatment of BRCA-mutated ovarian and breast cancers, are being investigated as a treatment option for TNBC patients with defects in DNA repair.
Furthermore, researchers can explore the use of immunotherapies for TNBC. Several clinical trials are currently investigating the efficacy of immune checkpoint inhibitors, such as pembrolizumab and atezolizumab, for the treatment of TNBC. These drugs work by blocking the interaction between cancer cells and immune cells, thereby enabling the immune system to recognize and attack cancer cells.
We’re Here Whenever You Need Us
info@encapsulate.bio
Phone
(two six seven) 290-7779
Open Hours
Mon – Fri: 8 am – 8 pm
Sat: 10 am – 4 pm
Address
400 Farmington Ave., Unit 1841, Farmington, CT